Our Research
Research Summary
Our lab focuses on uncovering the cellular and molecular mechanisms that drive neurodegeneration in the eye and, more broadly, throughout the central nervous system (CNS). We investigate how interactions among neurons, glial cells, and the neurovascular unit contribute to age-related damage in the retina and optic nerve. By understanding how these cellular relationships change with aging and disease, we aim to identify strategies to protect or restore visual function and, by extension, to promote neuronal survival across the brain.
Our research centers on three main areas:
Nitric oxide and soluble guanylate cyclase signaling as key modulators of neuronal physiology. We study how disruptions in this signaling pathway affect neuronal survival and neuroinflammatory responses, contributing to age-related neurodegeneration in both the eye and the brain.
The role of estrogen depletion in neuronal vulnerability within the CNS. Because neurodegenerative diseases become more prevalent in aging females, our work examines how the loss of estrogen alters cellular resilience, rendering neurons more susceptible to age-related stressors and degeneration.
Extracellular matrix remodeling and its impact on neuronal and glial health. In collaboration with the Calkins Laboratory, we investigate how age- and disease-related changes in the extracellular matrix, particularly in collagen composition, affect neuronal survival and glial function, and whether targeting these processes can enhance regeneration and resilience in neurodegenerative disease.
By identifying shared cellular stress responses and barriers to regeneration, we leverage the visual system as a model to reveal unifying mechanisms across the CNS and to translate insights from glaucoma research to other neurodegenerative disorders.
Publication Highlights
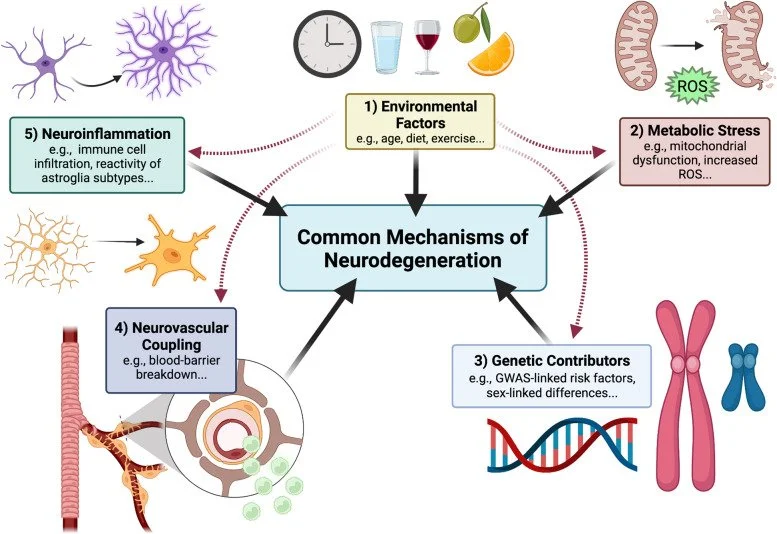
Solving neurodegeneration: common mechanisms and strategies for new treatments
Lauren K Wareham 1, Shane A Liddelow 2, Sally Temple 3, Larry I Benowitz 4, Adriana Di Polo 5, Cheryl Wellington 6, Jeffrey L Goldberg 7, Zhigang He 8, Xin Duan 9, Guojun Bu 10, Albert A Davis 11, Karthik Shekhar 12, Anna La Torre 13, David C Chan 14, M Valeria Canto-Soler 15, John G Flanagan 16, Preeti Subramanian 17, Sharyn Rossi 17, Thomas Brunner 18, Diane E Bovenkamp 17, David J Calkins 19
Across neurodegenerative diseases, common mechanisms may reveal novel therapeutic targets based on neuronal protection, repair, or regeneration, independent of etiology or site of disease pathology. To address these mechanisms and discuss emerging treatments, in April, 2021, Glaucoma Research Foundation, BrightFocus Foundation, and the Melza M. and Frank Theodore Barr Foundation collaborated to bring together key opinion leaders and experts in the field of neurodegenerative disease for a virtual meeting titled "Solving Neurodegeneration"
The Neurovascular Unit in Glaucomatous Neurodegeneration
Lauren K Wareham 1, David J Calkins 1
Glaucoma is a neurodegenerative disease of the visual system and leading cause of blindness worldwide. The disease is associated with sensitivity to intraocular pressure (IOP), which over a large range of magnitudes stresses retinal ganglion cell (RGC) axons as they pass through the optic nerve head in forming the optic projection to the brain. Despite clinical efforts to lower IOP, which is the only modifiable risk factor for glaucoma, RGC degeneration and ensuing loss of vision often persist.
Addressing neurodegeneration in glaucoma: Mechanisms, challenges, and treatments
Ghazi O Bou Ghanem 1, Lauren K Wareham 2, David J Calkins 3
Glaucoma is the leading cause of irreversible blindness globally. The disease causes vision loss due to neurodegeneration of the retinal ganglion cell (RGC) projection to the brain through the optic nerve. Glaucoma is associated with sensitivity to intraocular pressure (IOP). Thus, mainstay treatments seek to manage IOP, though many patients continue to lose vision. To address neurodegeneration directly, numerous preclinical studies seek to develop protective or reparative therapies that act independently of IOP.
Astrocyte Networks as Therapeutic Targets in Glaucomatous Neurodegeneration
Andrew M Boal 1, Michael L Risner 1, Melissa L Cooper 2 3, Lauren K Wareham 1, David J Calkins 1
Astrocytes are intimately involved in the response to neurodegenerative stress and have become an attractive target for the development of neuroprotective therapies. However, studies often focus on astrocytes as single-cell units. Astrocytes are densely interconnected by gap junctions that are composed primarily of the protein connexin-43 (Cx43) and can function as a broader network of cells.
Lauren K Wareham 1, Robert O Baratta 2, Brian J Del Buono 2, Eric Schlumpf 2, David J Calkins 1
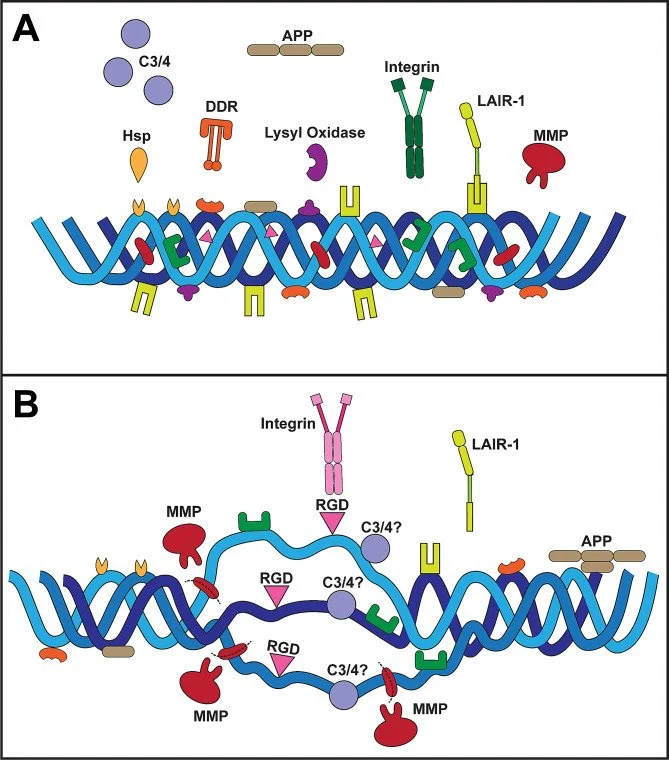
The extracellular matrix is a richly bioactive composition of substrates that provides biophysical stability, facilitates intercellular signaling, and both reflects and governs the physiological status of the local microenvironment. The matrix in the central nervous system (CNS) is far from simply an inert scaffold for mechanical support, instead conducting an active role in homeostasis and providing broad capacity for adaptation and remodeling in response to stress that otherwise would challenge equilibrium between neuronal, glial, and vascular elements.
Making tracks: microglia and the extracellular matrix
Lauren K Wareham 1, David J Calkins 2
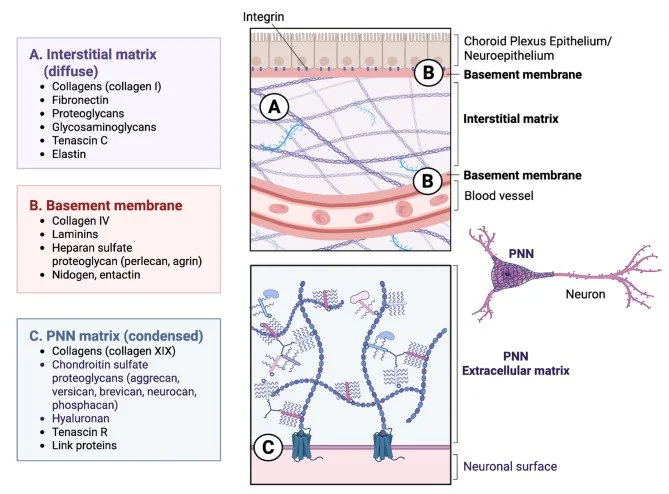
Microglia are resident immune cells of the central nervous system (CNS) and critical regulators of neural homeostasis, mediating immune surveillance, synaptic remodeling, debris clearance, and inflammatory signaling. Emerging evidence highlights the extracellular matrix (ECM) as important to microglial behavior in both physiological and pathological contexts. The CNS ECM is a dynamic and bioactive scaffold composed of three primary compartments: interstitial matrix, basement membranes at neurovascular and neuroepithelial interfaces, and perineuronal nets (PNNs).
Joseph M Holden 1 2, Olivia L Bossardet 1, Ghazi Bou Ghanem 1, David J Calkins 1, Lauren K Wareham 1
Astrocytes are the principle glial cells of the central nervous system and play an active role in maintaining proper metabolism in surrounding neurons. Because of their involvement in metabolic control, it is likely that their physiology changes in response to metabolic diseases such as diabetes and associated diabetic retinopathy. Here, we investigated whether microstructural changes in astrocyte morphology occur during the early stages of chronic hyperglycemia that may be indicative of early pathogenic programs.
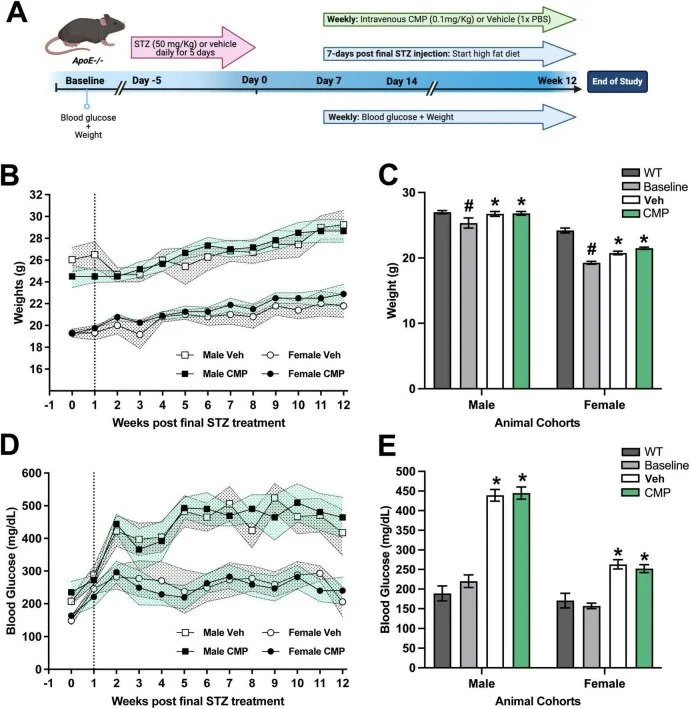
Collagen mimetic peptides as novel therapeutics for vascular disease in the central nervous system
Olivia L Bossardet 1, Joseph M Holden 1, Brian J Del Buono 2, Eric Schlumpf 2, Lauren K Wareham 1, David J Calkins 1
Loss of vascular integrity is a common comorbidity of neurodegenerative diseases of the central nervous system (CNS). Compromised blood flow to the brain and excessive vascular remodeling is evident in chronic systemic cardiovascular diseases such as atherosclerosis, driving neurodegeneration and subsequent cognitive decline. Vascular remodeling occurs in response to changes in the microenvironment, with the extracellular matrix (ECM) as a major component. Collagens within the ECM and vascular basement membrane are integral to endothelial cell (EC) function and maintenance of the blood-brain barrier. Disruption of the ECM and breakdown of collagen with disease may lead to vascular dysfunction and neurodegeneration.